How to Determine Which Solid Makes the Best Cathode
The cleanliness of the metal determines the uniformity of the electroplating. Construct solid-state-batteries by combining composite cathodes with a dense LLZO layer and a lithium anode.
These processes are.

. Attach bare copper wire to the cathode so that the object may be completely immersed in the plating solution. However in an electrolytic cell the anode is taken to be positive while the cathode is now negative. Determine processing parameters needed to reach 500 Whkg.
Porosity Total Volume Volume of the Solid Total Volume x 100. That designates the cathode. Thanks a lot for the help.
It has been found that Ni 3 ions are oxidized first on charging LiNi 1 y Co y O 2 in the 085 x 1 range while Co 3 ions are oxidized at a higher voltage. This seems reasonable as the anode is the source of electrons and cathode is where the electrons flow. On a battery the bumpy side is and the smooth side is -.
A larger percentage means that the rock has the ability to hold more water. It is well known that degradation and aging of LCOgraphite cells start with solid electrolyte interphase SEI formation and growth at the anode 56 but the end of life EOL is determined by the gradual development of a large resistance at the cathode 79 which increases and results into a power fade during cycling. The safe garnet-type solid electrolyte with the unique porous structure and surface coating to enable Li dendrite free cycling.
On a commercial battery the anode and cathode are clearly marked - for anode and for cathode. A larger percentage means that the rock has the ability to hold more water. C can be calculated by the calculating the amount of your active material devided by its Molecular weight then multiplied by x moles of Li.
The voltage is defined as zero for all temperatures. Any easy way to tell which end is the cathode vs. Make the cathode about ½ the max anode surface area.
Using this equation. From this determine the input voltages required to give the necessary Vgk values. Their discharge curves have a sloping form.
I want to calculate theoretical voltage of a Solid Oxide Fuel Cell fed by hydrogen 50 in mol at anode side and air at the cathode. The anode look for the silver stripe. Vi 8948V Because the cathode is already at 8948V above ground Vi- -12752V Because the grid needs to be 13V more negative Vi 8948 - -12752 217Vp-p Av 204217 094 Cathode followers can give some impressive voltage swings indeed.
12 cathode to anode. And surely the electric field strength or intensity is measured in Voltsmeters Vm. The potential or EMF of a cell is a measure of the tendency of the electrons to flow and is dependent on the difference in oxidizing or reducing strength of the two half-cells.
Put the red lead on the anode and the black lead on the cathode. In the past decade fruitful endeavors have been devoted to promoting each component of these batteries including solid electrolyte with high conductivity dendrite-free lithium anode and high-capacity cathode. As per the general definition an electrode is a substance that helps in the conduction of electricity wherein the electric current either enters or leaves the non-metallic medium like an electrolytic cell.
Make the cathode no larger than about 20 percent of the average anode surface area. The Electroplating is done at a current density of 15 mA cm 2 for 10 min. The diode is now forward biased meaning there is a current flowing through it.
Cathode materials based on solid solutions of LiNi 1 y Co y O 2 are less expensive than LiCoO 2 and show a larger discharge capacity at 4 V. Determine the masses of the anode and the cathode with the attached copper wire. Platinum which is inert to the action of the 1 M HCl is used as the electrode.
The one with the higher E is the better oxidizing agent reduced so it is in the cathode and the one with a lower E is the better reducing agent. When the circuit is complete electrons will flow spontaneously from the anode where oxidation is occurring to the cathode where reduction is occurring. Fundamental study and understanding of solid electrolyte-cathode interfaces regarding stability decomposition and resistances under a wide variety of synthesis processing and operating conditions.
The anode may be called the electron donor and the cathode the electron acceptor. Then Q 1510-3 600. When we see two ions elements without an chemical equation can we determine which element is a the anode and which is at the cathode by looking at their standard E.
Cu2aq 2e Cus 2 electrons are involved for every 1 copper atom so in the Nernst equation. In simple terms an electrode is a conductor that helps in establishing electrical contact. The photo just to clarify the two platesvoltage across them the photo from Capacitance Charging.
A ratio of 110 is often used. Make the cathode between ¾ - 1 times the max anode area about 113 up to 11. Hydrogen gas at 1 atm is bubbled through 1 M HCl solution.
Solid-state lithium batteries have aroused wide interest with the probability to guarantee safety and high energy density at the same time. 15 cathode to anode. If youre setting up a galvanic cell youll need to keep the redox reaction in mind to identify the electrodes.
E cathode cathode efficiency of current that deposits the metal F faradays constant 96485 A seconds My question is how to calculate Wt for example in the case of tin plating SnSO4 in the presence of H2SO4 acid medium. E is the standard reduction potentialThe superscript on the E denotes standard conditions 1 bar or 1 atm for gases 1 M for solutes. If you know what units you are working with you dont need any equations.
The electric field strength between two plates one positive and another negative is given as E V d where V is the voltage between the two electrodes and d is the distance between the plates. In a galvanic voltaic cell the anode is considered negative and the cathode is considered positive. Electrons on the surface of the electrode.
Sometimes only the terminal is marked. To determine the voltage of any cell subtract the electrode potential of the anode from that of the cathode and you get the electrode potential of the cell or voltage. How can i do it.
Before we learn about cathode and anode we need to first understand what an electrode is. These electrons flow from the anode to the cathode. The anode is the positive end while the cathode is the negative end.
Ecell E cell RT nF lnQ we have that n 2 mols e 1 mol Cu2 while the Faraday constant is F 96500 Cmol e. Make your mass measurements to the nearest 01 mg. Energy density cathode materials into the porous scaffolds.
In a Galvanic cell the anode is.

Summary Of The Most Common Cathode And Anode Materials Performance Download Scientific Diagram
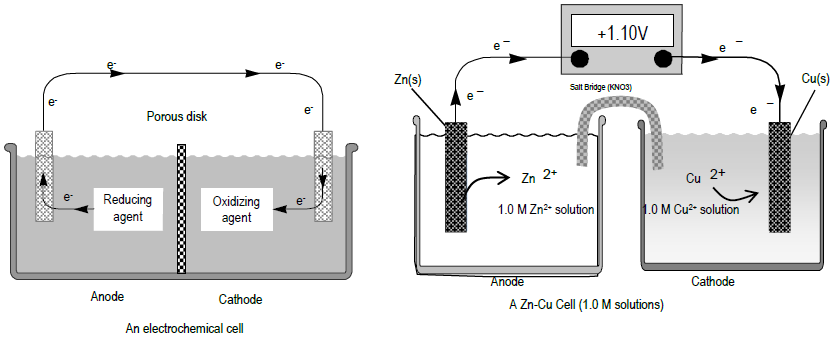
Lab 13 Electrochemistry And The Nernst Equation

Electrochemistry Why Is Mass Gained At The Cathode Chemistry Stack Exchange
No comments for "How to Determine Which Solid Makes the Best Cathode"
Post a Comment